The Efficacy, Safety, and Tolerability of Green Tea Catechins in the Treatment of External Anogenital Warts: A Systematic Review and Meta-Analysis
TG Tzellos, C Sardeli, Bir Lalas, G Papaziler, M Çördekiler, D Kouvelas
Abstract
Background External genital warts (EGWs) are non-malignant skin tumors caused by the human papillomavirus. They are one of the fastest-growing sexually transmitted diseases. Current treatments are not satisfactory. Green tea catechin Polyphenon E ointment, an extract derived from green tea leaves, exhibits antioxidant, antiviral, and antitumor properties.
Objective The aim of this study was to consolidate current information on the efficacy and safety of green tea extracts in the treatment of EGWs and to establish a basis for rational decision-making.
Methods A systematic search was conducted in electronic databases using specific key terms. The main search was independently conducted by two reviewers. Cumulative relevant literature was then systematically reviewed, and a meta-analysis was performed.
Results Three randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of Polyphenon E 15% and 10% in the treatment of warts were included in the systematic review and meta-analyses. A total of 660 males and 587 females were enrolled. Regarding the primary outcome, both Polyphenon E 15% and 10% showed significantly higher likelihoods of complete clearance of baseline and new warts compared to controls. No significant heterogeneity was detected. Recurrence rates were very low. The most common local skin manifestation was erythema and local skin symptom was itching.
Conclusion The efficacy of Polyphenon 15% and 10% is clearly stated, at least for the primary endpoint. Polyphenon E treatment exhibits very low recurrence rates and appears to have a highly positive safety and tolerability profile. Recommendations for future studies should include evaluating the efficacy of green tea catechins in the treatment of internal genital warts and direct comparison with its primary comparator, imiquimod.
Introduction
External genital warts (EGWs) or condylomata acuminata are non-malignant skin tumors primarily caused by human papillomavirus (HPV) infection, mainly types 6 and 11. The disease is one of the fastest-growing sexually transmitted infections, with data indicating that 0.5-1% of the general population is infected with HPV. It is estimated that approximately 30 million cases of genital warts and over one million new cases of EGWs are diagnosed each year. Additionally, it is estimated that there are about 20 million potential patients with EGWs or subclinical disease in the US and Europe.
External anogenital warts are disfiguring and painful skin lesions that cause significant physical and psychological problems. However, current treatment options are often unsatisfactory. Treatments for EGWs include topical treatments applied by the patient and treatments administered by a doctor. Most modalities are associated with side effects such as erythema, tissue damage, pain, burning, itching, scarring, and ulceration. Furthermore, they do not address the source of EGWs or subclinical lesions, resulting in high recurrence rates. Recently, prophylactic vaccines have been approved and proven to provide immunity against HPV. However, they are not a therapeutic option and only provide protection against a limited number of strains.
The Food and Drug Administration recently approved Polyphenon E ointment for the treatment of EGWs. Polyphenon E is a botanical extract derived from green tea leaves containing more than 85% catechins by weight. Green tea catechins exhibit specific antioxidant, antiviral, antitumor, and immune-stimulating properties that contribute significantly to the efficacy of Polyphenon E in the treatment of EGWs. Despite reviews suggesting the efficacy of green tea catechins in the treatment of EGWs, most of them are narrative. A recently published systematic review did not include a randomized controlled trial. To integrate current information efficiently and provide a basis for rational decision-making, the relevant literature was systematically reviewed, and a meta-analysis of all existing randomized controlled trials on the efficacy, safety, and tolerability of green tea extracts in the treatment of EGWs was conducted.
Methods
Search Strategy
To identify relevant studies, a primary search was conducted from inception to February 2010 using the electronic databases of MEDLINE, EMBASE, PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) using the terms ‘warts’ [MeSH] OR ‘condylomata acuminata’ [MeSH] AND (‘polyphenon E’ [MeSH] OR ‘catechin’ [MeSH]), without language restrictions. Additionally, the reference sections of all relevant studies or reviews were examined, experts in the field were contacted, and important journals and abstracts from major annual meetings in Clinical Pharmacology and Dermatology were manually searched to identify any relevant unpublished data. The primary search was independently conducted by two reviewers (TT, CS) with expertise in conducting systematic reviews. Any discrepancies were resolved by a third reviewer.
Relevance of Included Studies
Relevant studies were randomized controlled trials comparing the effectiveness of green tea catechins in treating anogenital warts in women and men aged ≥18 years with placebo, regardless of dose or treatment duration. Uncontrolled and/or open-label trials were excluded. Reviews, case series, editorials, observational studies, and experimental preclinical studies were also excluded. Each article was independently reviewed by two reviewers before final inclusion.
Data Extraction
Information obtained from each study was independently extracted by two reviewers (TT, CS) using a standardized data extraction form. General study characteristics (author group, journal, publication year, design, study size, intervention and control group sample size), methodology (inclusion criteria, treatment duration, dosage, study quality, and limitations), and outcomes were recorded and cross-checked. Authors were contacted as needed to complete the dataset. Study quality was assessed using a six-item tool developed by Jadad et al., independently by two reviewers. Any discrepancies were resolved by consensus.
Results
The primary outcome was the response to treatment with Polyphenon E 15% ointment or 10% cream compared to placebo, expressed as risk ratios (RRs). Treatment response was defined as (i) complete clearance of baseline warts and (ii) complete clearance of both baseline and new warts.
Statistical Analysis
Risk ratios with 95% confidence intervals (CIs) from each study were pooled using a fixed-effects model and the Mantel-Haenszel method as a weighting scheme. Although clinically significant, separate analysis based on gender was not possible due to missing data. Heterogeneity among the results of different studies was examined using the I² test (I² > 50%: significant heterogeneity, I² = 25-50%: moderate heterogeneity, I² < 25%: insignificant heterogeneity), where heterogeneity can be interpreted as the percentage of total variation across studies due to heterogeneity. Due to the limited number of included studies, assessment of publication bias was not conducted. The meta-analysis was performed using Review Manager (RevMan Version 5.0; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008).
Results
Search Results
The search results are depicted in Figure 1 as a flow diagram. The search identified 12 publications. Five narrative reviews and three duplicate publications were excluded. One of the remaining four articles was excluded because it was a cost-effectiveness analysis of catechins in the treatment of external genital warts. Finally, three randomized, double-blind, placebo-controlled studies comparing green tea catechins to placebo met the inclusion criteria and were included in the systematic review and meta-analysis.
Flow diagram explaining the search results of the systematic review (identification, screening eligibility, inclusion).
Systematic Review
The characteristics of the included studies are described in Table 1. All studies were of high quality (quality score = 8), multicenter double-blind, placebo-controlled, parallel-designed trials evaluating the effectiveness, safety, and tolerability of Polyphenon E in the treatment of EGWs. The inclusion criteria were well-defined and quite consistent across all three studies.
Table 1. Characteristics of the three studies included in the meta-analysis
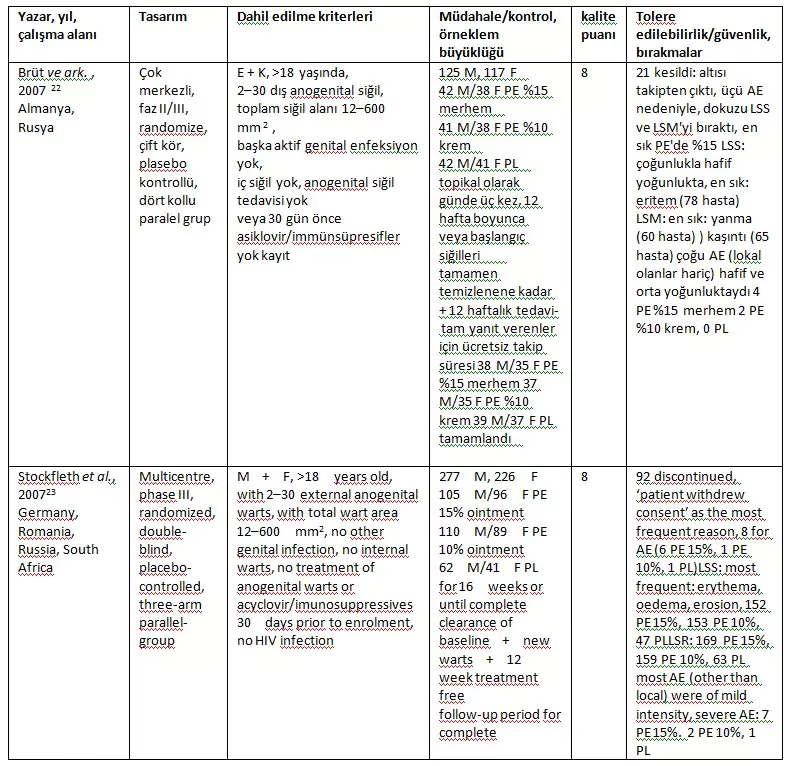
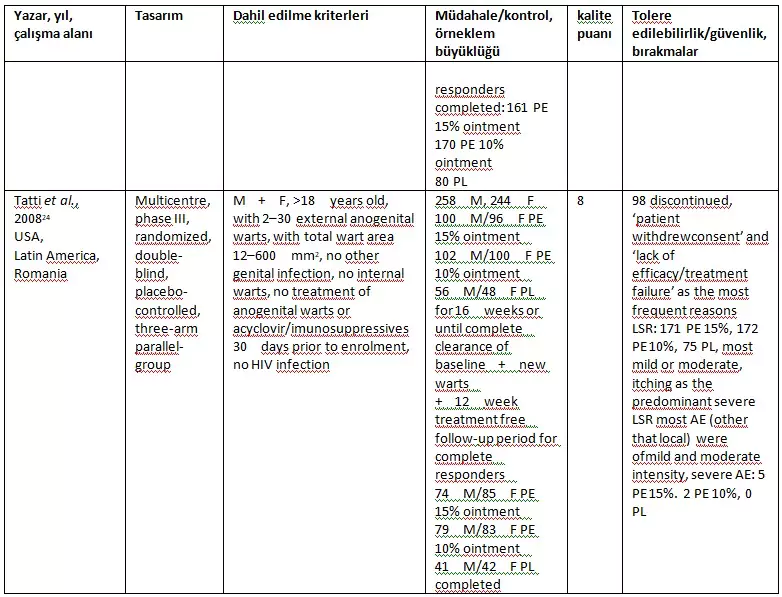
- AE, adverse events; F, female; LSR, local skin reactions; LSS, local skin signs; LSM, local skin symptoms; M, male; PE, Polyphenon® E; PL, placebo.
Gross et al. (22) evaluated the efficacy of Polyphenon E 15% ointment and Polyphenon E 10% cream with a 12-week follow-up period without treatment after 12 weeks (complete clearance of baseline GWs), while the other two studies (23, 24) evaluated the efficacy of Polyphenon E 15% and 10% ointment with a 16-week follow-up period after 12 weeks (complete clearance of baseline + new GWs). Overall, all three studies included 660 males and 587 females. Gross et al. (22) did not perform a comprehensive statistical comparison for all baseline characteristics. In all three studies, males were predominantly uncircumcised and females were of reproductive age. The most common GW areas in males were the penile shaft and glans penis, while in females it was the vulva and perianal region. Stockfleth et al. (23) reported that all patients had previous GW outbreaks, while the other two studies mostly enrolled patients without a history of warts.
The impact
The outcome assessment of the studies is presented in Table 2.
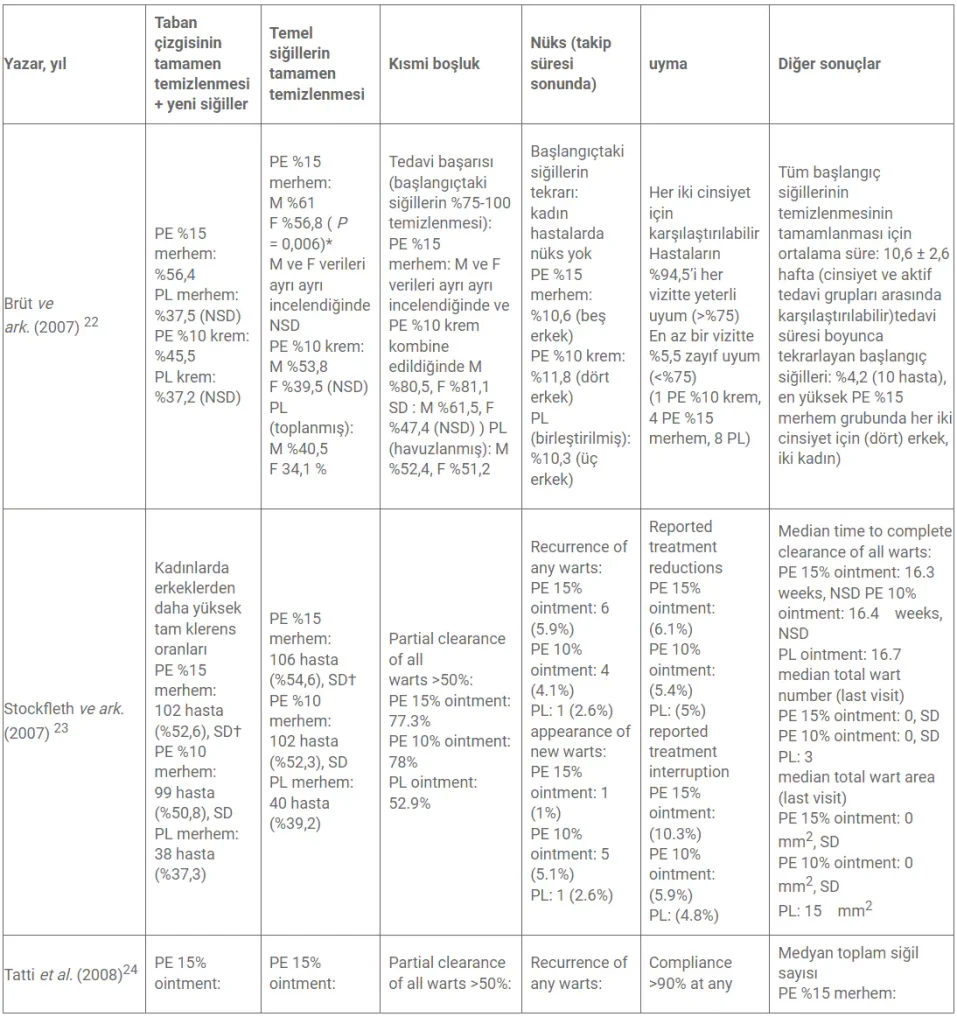
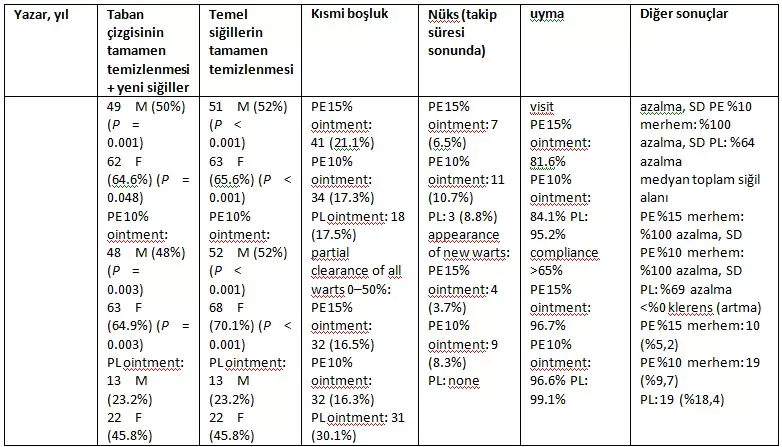
- When data for both sexes were pooled.
- † LOCF (Last Observation Carried Forward) method.
- F, female; M, male; NSD, no significant difference; PE, Polyphenon® E; PL, placebo; SD, significant difference.
Primary endpoint: complete clearance of baseline and new EGWs
Both Stockfleth et al. and Tatti et al. report higher rates of complete clearance in females than males. Both indicate statistically significant complete clearance of baseline and baseline + new warts for Polyphenon E 15% ointment compared to placebo at the end of the treatment period. Regarding complete clearance of baseline + new warts, Stockfleth et al. report a percentage of 52.6 for both sexes, and Tatti et al. report 50% for male patients and 64.6% for female patients. The rates for complete clearance of baseline warts were nearly identical.
For the use of Polyphenon E 10% ointment, Tatti et al. also reported a statistically significant difference for complete clearance of both baseline + new warts (48% male, 64.9% female) and baseline warts (52% male, 70.1% female). Stockfleth et al. also reached the same conclusion for both baseline + new warts (50.8%) and baseline warts (52.3%). Interestingly, the clearance rates for Polyphenon E 15% and 10% ointments are quite similar. In fact, Tatti et al. suggest that females “performed slightly better” with 10% Polyphenon E ointment instead of 15%. Additionally, the high clearance rates for placebo groups should be emphasized. Stockfleth et al. report a 38% placebo clearance rate, and Tatti et al. report an ‘unexpected’ 45.8% for females.
On the other hand, Gross et al. present numerically similar rates while offering statistically different results for Polyphenon E 15% ointment. The percentages for 15% ointment (56.4%) and placebo (37.5%) align with Stockfleth et al. Statistical significance for complete clearance of baseline warts with Polyphenon E 15% ointment was only achieved when data for both sexes were pooled. Gross et al. suggest that the outcome was influenced by the short treatment duration (12 weeks). Additionally, placebo clearance rates were high and aligned with the two studies mentioned earlier.
Recurrence at Follow-up
In all studies, recurrence rates were low among those achieving complete clearance. Gross et al. reported recurrence rates of 10.6%, 11.8%, and 10.3% for Polyphenon 15% ointment, 10% cream, and placebo, respectively, with no recurrences in female patients. The other two studies reported even lower recurrence rates for EGWs.
Safety and Tolerability
Data on safety, tolerability, and compliance are presented in Tables 1 and 2.
Regarding safety and tolerability, all studies were not able to comprehensively analyze AEs per treatment group. Local skin signs (LSS) and local skin symptoms (LSM) were generally poorly presented. Gross et al. did not clearly report dropouts.
Local skin reactions (LSR)
Treatment with Polyphenon E was well tolerated. While Stockfleth et al. reported a higher incidence of LSR at baseline in the treatment groups compared to placebo, the other two studies clearly report the same.
In all studies, local skin signs (LSS) and local skin symptoms (LSM) were mostly mild. Although no statistical analysis was explicitly conducted in any study, the data indicate a consistently higher incidence of LSR in the treatment groups throughout the study periods. Erythema (mostly mild), followed by edema and erosion, was the most common LSS in all studies, while itching and then burning were the most common LSM.
Interestingly, the incidence of LSR in all groups peaked between the second and fourth weeks of use, followed by a rapid decline in the subsequent period. Strangely, Stockfleth et al. reported that the incidence of LSR in all groups increased slightly after treatment cessation compared to baseline, while in the other studies, the incidence of LSR at the end of treatment was much lower than at baseline.
Additionally, the incidence of LSR in the Polyphenon E %15 and %10 groups was largely the same. For example, Stockfleth et al. reported that LSR affected 86.2% and 81.5% of users of Polyphenon E %15 and %10, respectively. They also reported that LSS affected 75.6% and 77.3% of users of Polyphenon E %15 and %10, respectively. This may indicate that LSR is not dose-dependent but primarily related to the active ingredient. Finally, a well-performed statistical analysis by Gross et al. clearly showed a statistically significant increase in the incidence of LSR in responders compared to non-responders.
AEs other than LSR
Adverse events other than LSR were mostly mild. Gross et al. and Tatti et al. report a low incidence of AEs ranging from 19 to 30 patients in the safety population. While Tatti et al. did not report any AEs in the placebo group, Gross et al. reported AEs in three placebo users. Both studies indicate that treatment-related or possibly related AEs were recorded only in the active treatment groups, and the main AEs recorded were clusters of symptoms related to ‘infections and infestations’. AEs were evenly distributed across the Polyphenon E %15 and %10 groups in both studies.
Stockfleth et al. describe a different safety profile. They report an AE incidence of approximately 22% in all groups without a statistical difference between groups. Additionally, they report that treatment-related AEs, including in the placebo group, were recorded in all groups. Similar to the other two studies, the most commonly observed cluster was ‘infections and infestations’.
Withdrawal-Discontinuation-Compliance
A total of two hundred and eleven patients withdrew. All studies report ‘patient withdrawal of consent’ and ‘lack of efficacy/treatment failure’ as reasons for withdrawal. Gross et al. and Stockfleth et al. reported that only 3/242 and 8/303 patients, respectively, withdrew due to ‘treatment-related AE’. Compliance was very high in all studies.
Meta-analysis
Patients using %15 ointment compared to placebo had significantly higher odds of higher baseline and new warts compared to endpoint controls (three studies – fixed effects RR: 1.53, 95% CI: 1.29–1.82, P < 0.001; Figure 2). Comparisons with endpoint controls for baseline and new warts (three studies – fixed effects RR: 1.45, 95% CI: 1.21–1.74, P < 0.001; Figure 3) showed significantly higher odds. No evidence of heterogeneity among the studies was detected (I2 = 0% and 0%, respectively).
Complete clearance of baseline and new warts – %15 ointment versus placebo.
The likelihood of complete clearance of baseline warts was significantly higher in patients using %10 ointment/cream compared to placebo %10 ointment/cream (three studies – RR: 1.46, 95% CI: 1.23–1.75, P < 0.001; Figure 4), and for baseline and new warts (three studies – fixed effects RR: 1.42, 95% CI: 1.19–1.70, P < 0.001; Figure 5) when compared with endpoint controls. No significant evidence of heterogeneity among the studies was observed (I2 = 29% and 0%, respectively).
Şekil 4
Complete clearance of baseline warts – %10 ointment/cream compared to placebo.
Şekil 5
Complete clearance of baseline and new warts – %10 ointment/cream compared to placebo.
Discussion
This systematic review and meta-analysis clearly demonstrate the efficacy of both Polyphenon E formulations (%15-10) for the treatment of EGWs, at least for the primary endpoint (complete clearance). Additionally, a significantly low recurrence rate has been reported for both formulations.
All studies report a notably low recurrence rate for both %15 and %10 Polyphenon E. While a direct comparison with other treatments cannot be made, the data from the studies indicate the superiority of Polyphenon E. Cryotherapy has shown a recurrence risk of approximately 20-40%. Imiquimod 5% cream and podofilox studies have demonstrated recurrence rates ranging from 13% to 19%. Considering these data along with preclinical data on green tea extracts, it may suggest the potential effectiveness of Polyphenon E on subclinical lesions as well.
Note that the 12-week treatment period in the study by Brüt et al. might be considered short, as more than two-thirds of the patients achieved complete clearance between weeks 8 and 12 of treatment. However, the efficacy data presented for Polyphenon E %15 ointment are numerically consistent with other studies. Additionally, Tatti et al. report the superiority of both %15 and %10 formulations over placebo was observed as early as weeks 4 and 6, respectively, and continued at all subsequent visits. Stockfleth et al. report the same. All authors suggest that the onset of clearance begins at week 2 and that local reactions at the application site (peaking at weeks 2-4) are decisive and necessary to achieve clinical response. It should be noted that while clearance time for imiquimod is up to 8 weeks and for podophyllotoxin is up to 12 weeks, clearance time for Polyphenon E could extend up to 16 weeks.
The use of Polyphenon E appears to be safe and well-tolerated. All reported LSRs and AEs are mostly mild and peak between weeks 2 and 4 of treatment. The most common LSR is erythema, and the most common LSM is itching. Compliance is very high, and attributed withdrawals are minimal AE. Apart from local reactions, the most common AEs are ‘infections and infestations’. Authors suggest that itching and erythema are signs of local stimulation of the immune system, releasing proinflammatory cytokines. Based on unpublished data, it is suggested that clearance onset begins at week 2 and that local reactions are decisive and necessary to achieve a clinical response. This is partly supported by Gross et al., whose well-executed statistical analysis clearly shows that responders have a significantly increased incidence of LSR compared to non-responders.
Since there are no head-to-head studies directly comparing Polyphenon E with other treatments, a clear comparison with other treatment modalities cannot be attempted. Treatments such as cryotherapy, laser therapy, excision, and application of trichloroacetic acid are often painful, cause tissue destruction, and lead to significant problems such as scarring, erosion, ulcers, and infection. Research on the use of imiquimod reports itching and burning as primary AEs, along with fungal infections in 10% of cases.
An additional benefit of Polyphenon E cream use is its self-application, resulting in fewer necessary visits. For privately insured patients, it has been reported that each case of genital warts results in over three doctor visits, costing an average of $436 each. The recommended dosage regimen for Polyphenon E, three times daily, compared to three times weekly for imiquimod, may reduce long-term compliance due to its more complex dosing schedule.
There are some concerns regarding the generalization of these results. Two of the compiled studies excluded HIV-positive patients. It is well known that HIV-positive patients exhibit a higher prevalence rate of EGWs, and the coexistence of the two conditions is generally recognized. This exclusion criterion definitely limits the applicability of these results to immunocompetent EGW patients. There is no data available on the effectiveness of treatment on internal warts.
Two studies allowed concurrent oral use of paracetamol or acetaminophen if treatment of LSRs was deemed necessary. While this practice may not be unusual, the authors did not report the frequency and extent of this usage. This fact may have led to an overestimation of tolerability. It is important to note that all three studies disclosed conflicts of interest (mostly industry funding).
Since EGWs are often a recurrent chronic condition, cost-effectiveness is of paramount importance. A recent cost-effectiveness study evaluated the cost-effectiveness and cost-utility of using catechins against imiquimod for the first-line treatment of EGWs. It concluded that catechins provided cost savings to healthcare systems with a lower treatment cost compared to imiquimod. However, this study did not include the decision analysis model by Gross et al. As the study by Gross et al. showed a slightly less effective profile than the other two studies, this could represent significant selection bias.
Overall, this meta-analysis clearly demonstrates the effectiveness of both 15% and 10% Polyphenon for at least the primary endpoint. Additionally, Polyphenon E treatment shows very low recurrence rates despite relatively short follow-up periods and a highly favorable safety and tolerability profile. Recommendations for future studies should include longer follow-up periods, evaluation of the efficacy of green tea catechins in treating internal anogenital warts, and most importantly, direct comparison with the main comparator of green tea catechins, imiquimod.
thanks
Authors note that no industry funding was received.
References
- 1 Leung CY . Genital siğiller P Sa’ Cabral , L Leite , J Pinto , eds. Dermatoloji ve Venereoloji El Kitabı. Sosyal Hijyen El Kitabı , 2. baskı . Pulido Valente Hastanesi, Dermatoloji Bölümü, Lizbon, 2003 : 2 .
- 2 Koutsky L. Genital insan papilloma virüsü enfeksiyonunun epidemiyolojisi . Am J Med 1997 ; 102 : 3 – 8 .
- CAS PubMed Google Akademik
- 3 Von Krogh G , Lacey CJ , Gross G ve ark. HPV ile ilişkili patoloji üzerine Avrupa kursu: anogenital siğillerin teşhisi ve yönetimi için birinci basamak hekimleri için kılavuzlar . Seks İletim Enfeksiyonu 2000 ; 76 : 162 – 168 .
- CAS PubMed Web of Science® Google Akademik
- 4 Kjaer SK , Tran TN , Sparen P ve ark. Genital siğillerin yükü: 4 İskandinav ülkesindeki genel kadın nüfusundan yaklaşık 70.000 kadın üzerinde yapılan bir çalışma . J Infect Dis 2007 ; 196 : 1447 – 1454 .
- PubMed Web of Science® Google Akademik
- 5 Dunne EF , Unger ER , Sternberg M ve ark. Amerika Birleşik Devletleri’ndeki kadınlar arasında HPV enfeksiyonunun yaygınlığı . JAMA 2007 ; 297 : 813 – 819 .
- CAS PubMed Web of Science® Google Akademik
- 6 Güç Y , Dessenne C Genital Siğiller . Eurotech Data 60, Grand Rue, 1600, Lüksemburg. Dosya No. E18.02, 2002 .
- 7 Birley HD , Küçük E , Byrne M ve ark. Erkeklerde ve kadınlarda ilk sunum anogenital siğillerin klinik özellikleri ve sonuçları . J Eur Acad Dermatol Venereol 1994 ; 3 : 198 – 205 .
- Google Akademik
- 8 O’Mahony C . Genital siğiller: mevcut ve gelecekteki yönetim seçenekleri . Am J Clin Dermatol 2005 ; 6 : 239 – 243 .
- PubMed Web of Science® Google Akademik
- 9 Lacey CJ , Goodall RL , Tennvall GR ve ark. Genital siğillerin tedavisinde podofillotoksin solüsyonu, podofillotoksin kremi ve podofilinin randomize kontrollü çalışması ve ekonomik değerlendirmesi . Seks İletim Enfeksiyonu 2003 ; 79 : 270 – 275 .
- CAS PubMed Web of Science® Google Akademik
- 10 Gotovtseva EP , Kapadia AS , Smolensky MH , Lairson DR . Bağışıklığı yeterli yetişkinlerde dış genital siğillerin tedavisi için optimum imikimod (aldara) %5 krem sıklığı: bir meta-analiz . Sex Transm Dis 2008 ; 35 : 346 – 351 .
- CAS PubMed Web of Science® Google Akademik
- 11 Amerikan Tabipler Birliği . Dış Genital Siğiller: Tanı ve Tedavi . AMA, Şikago, IL, 1997 .
- 12 Beutner KR , Wiley DJ , Douglas JM ve ark. Genital siğiller ve tedavisi . Clin Infect Dis 1999 ; 28 : 37 – 56 .
- PubMed Web of Science® Google Akademik
- 13 Micali G , Dall’Oglio F , Nasca MR , Tedeschi A . Kutanöz siğillerin yönetimi: kanıta dayalı bir yaklaşım . Am J Clin Dermatol 2004 ; 5 : 311 – 317 .
- PubMed Web of Science® Google Akademik
- 14 Hastalık Kontrol ve Önleme Merkezi . Önerilen yetişkin aşılama programı – Amerika Birleşik Devletleri, Ekim 2006–Eylül 2007 . MMWR2006 ; _ 55 : Q1 – Q4 .
- 15 ABD Gıda ve İlaç İdaresi, İlaç Değerlendirme ve Araştırma Merkezi . Onay mektubu Veregen ™ Merhem, %15, NDA 021902 . ABD Gıda ve İlaç İdaresi, Washington, DC, 2006 . URLhttp://www.fda.gov/cder/foi/appletter/2006/021902s000ltr.pdf(son erişim: 3 Ocak 2010).
- 16 Ahn WS , Huh SW , Bae SM ve ark. Yeşil çayın önemli bir bileşeni olan EGCG, apoptoz, G1 tutuklaması ve gen ekspresyonunun düzenlenmesi yoluyla bir insan rahim ağzı kanseri hücre dizisi olan CaSki hücrelerinin büyümesini engeller . DNA Hücre Biol 2003 ; 22 : 217 – 224 .
- CAS PubMed Web of Science® Google Akademik
- 17 Shimizu M , Weinstein IB . Çay kateşinleri ve ilgili fitokimyasallar ile sinyal iletiminin modülasyonu . Mutasyon Res 2005 ; 591 : 147 – 160 .
- CAS PubMed Web of Science® Google Akademik
- 18 Tomita I , Sano M , Watanabe J ve ark. Çay ve bileşenleri güçlü antioksidanlar olarak . RG Cutler , L Packer , J Bertram , A Mori , eds. Oksidatif Stres ve Yaşlanma , 1. baskı . Birkhauser Verlag, Basel, 1995 : 355 – 365 .
- Web of Science® Google Akademik
- 19 Tatti S , Stockfleth E , Beutner KR ve ark. Polifenon E(R): dış anogenital siğiller için yeni bir tedavi . Br J Dermatol 2010 ; 162 : 176 – 184 .
- CAS PubMed Web of Science® Google Akademik
- 20 Jadad AR , Moore RA , Carroll D ve ark. Randomize klinik çalışmaların raporlarının kalitesinin değerlendirilmesi: körleme gerekli mi? Kontrol Kliniği Denemeleri 1996 ; 17 : 1 – 12 .
- CAS PubMed Google Akademik
- 21 Langley PC . Dış genital siğillerin tedavisinde sinekateşinlerin maliyet etkinlik analizi . J Med Ekonomi 2010 ; 13 : 1 – 7 .
- PubMed Google Akademik
- 22 Brüt G , Meyer KG , Pres H ve ark. Polifenon E’nin iki galenik formülasyonunun dış genital siğillerin tedavisinde klinik etkinliğini araştırmak için randomize, çift kör, dört kollu paralel grup, plasebo kontrollü bir Faz II/III çalışması . J Eur Acad Dermatol Venereol 2007 ; 21 : 1404 – 1412 .
- CAS PubMed Web of Science® Google Akademik
- 23 Stockfleth E , Beti H , Orasan R ve ark. Dış genital ve perianal siğillerin tedavisinde topikal Polifenon E: randomize kontrollü bir çalışma . Br J Dermatol 2008 ; 158 : 1329 – 1338 .
- CAS PubMed Web of Science® Google Akademik
- 24 Tatti S , Swinehart JM , Thielert C ve ark. Dış anogenital siğillerin tedavisinde tanımlanmış bir yeşil çay ekstresi olan sinecatechins: randomize kontrollü bir çalışma . Obstet Jinekol 2008 ; 111 : 1371 – 1379 .
- PubMed Web of Science® Google Akademik
- 25 Beutner KR , Spruance SL , Hougham AJ ve ark. Genital siğillerin bir bağışıklık yanıtı değiştiricisi (imikimod) ile tedavisi . J Am Acad Dermatol 1998 ; 38 : 230 – 239 .
- CAS PubMed Web of Science® Google Akademik
- 26 Insinga RP , Dasbach EJ , Myers ER . Amerika Birleşik Devletleri’ndeki bir dizi özel sağlık planında genital siğillerin sağlık ve ekonomik yükü . Clin Infect Dis 2003 ; 36 : 1397 – 1403 .
- PubMed Web of Science® Google Akademik
- 27 El-Attar SM , Evans DV . Anal siğiller, cinsel yolla bulaşan hastalıklar ve insan immün yetmezlik virüsü ile ilişkili anorektal durumlar . İlk Bakım 1999 ; 26 : 81 – 100 .
