Sara M. Meltzer MD, Bradley J. Monk MD ve Krishnansu S. Tewari MD
American Journal of Obstetrics and Gynecology, March 1, 2009, Volume 200, Issue 3, Pages 233.e1-233.e7, Copyright © 2009 Mosby, Inc.
This review evaluates the antiviral, antioxidant, and immune-stimulating properties of green tea catechins. Two randomized studies assessing the effectiveness of green tea catechins in the treatment of external genital warts are presented, and the reported side effects associated with this topical treatment method are summarized. Finally, the mechanism of action, wart clearance rate, clearance time, and toxicity profile of green tea catechins are compared with those of the other two patient-applied topical agents approved for the treatment of anogenital warts, podofilox and imiquimod.
Each year, more than 1 million new cases of anogenital warts are diagnosed in the United States alone. Also known as condyloma acuminatum, these warts are caused by infection with the human papillomavirus (HPV). The spectrum of diseases attributed to HPV infection ranges from genital warts and potentially fatal neonatal laryngeal papillomatosis (caused by HPV subtypes 6 and 11) to dysplastic and frankly invasive carcinomas of the cervix, vagina, vulva, and anus (primarily caused by the oncogenic HPVs 16 and 18). Historically, the treatment of anogenital warts has required chemical destruction, immunologic therapy, and/or surgical procedures; many patients have been treated with multiple therapeutic modalities, often leading to excessive scarring and disfigurement and ultimately resulting in disease recurrence. Recently, the U.S. Food and Drug Administration (FDA) approved green tea sinecatechins, Polyphenon E (Veregen) ointment, for the treatment of anogenital warts in both men and women. This review will highlight the biological properties of catechins, report on clinical trials of Polyphenon E ointment in the treatment of anogenital warts, and demonstrate the efficacy of this botanical through case presentations.
The Discovery of the Medicinal Properties of Teas
Archaeological evidence suggests that tea plant culture likely originated in China more than 5,000 years ago and was brought from there to India, Japan, Thailand, Korea, and Sri Lanka. The medicinal properties of tea leaves first appeared in a Chinese book on pharmaceutical plants (circa 200 BCE). Later, in Kissa Yojoki (The Book of Tea, circa 1191), tea was listed as a remedy for controlling bleeding, helping heal wounds, regulating body temperature, controlling blood sugar, and aiding digestion. Green tea, which accounts for 20% of the tea produced worldwide, is probably the most consumed beverage in Asian society outside of water (Figure 1). The main substances found in green tea are caffeine (2-4%), amino acids (4%), lignin (6.5%), organic acids (1.5%), protein (15%), chlorophyll (0.5%), and polyphenols (25-35%). The beneficial effects of green tea are attributed to polyphenolic compounds, which are essentially colorless. Epigallocatechin gallate (EGCG) and epigallocatechin (EGC) are the most important polyphenols (Figure 2), and one cup of green tea contains about 300-400 mg of polyphenols or 10-30 mg of EGCG. Due to differences in processing tea leaves after harvesting, catechins are found in higher amounts in green tea than in black or oolong tea.
Polyphenon E (Veregen) ointment is a botanical drug containing more than 85% catechins, approved by the FDA for the topical treatment of anogenital warts (Veregen ointment, 15% [NDA 21-902, October 31, 2006]). These catechins are a partially purified fraction of the water extract of green tea leaves obtained from Camellia sinensis (L.) O Kuntze. Polyphenon E (Veregen) ointment is the first and currently the only FDA-approved botanical for the treatment of human disease. Its established efficacy in destroying anogenital warts is due to antiviral, immune-stimulating, and antioxidant mechanisms.
Biological Properties of Green Tea Catechins
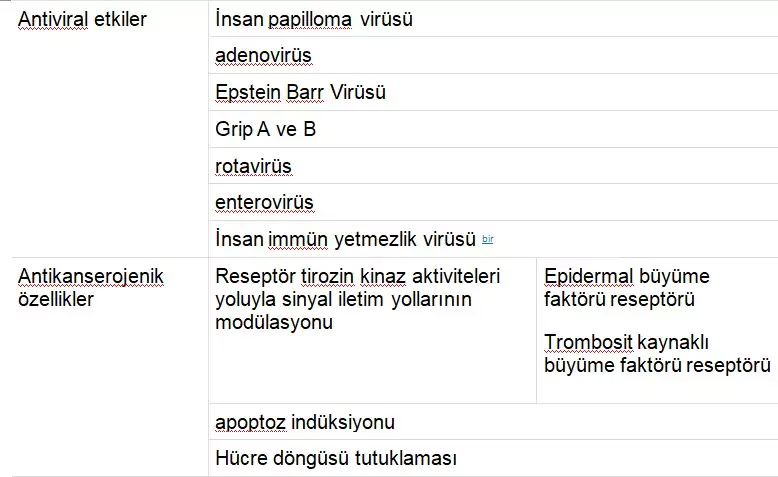
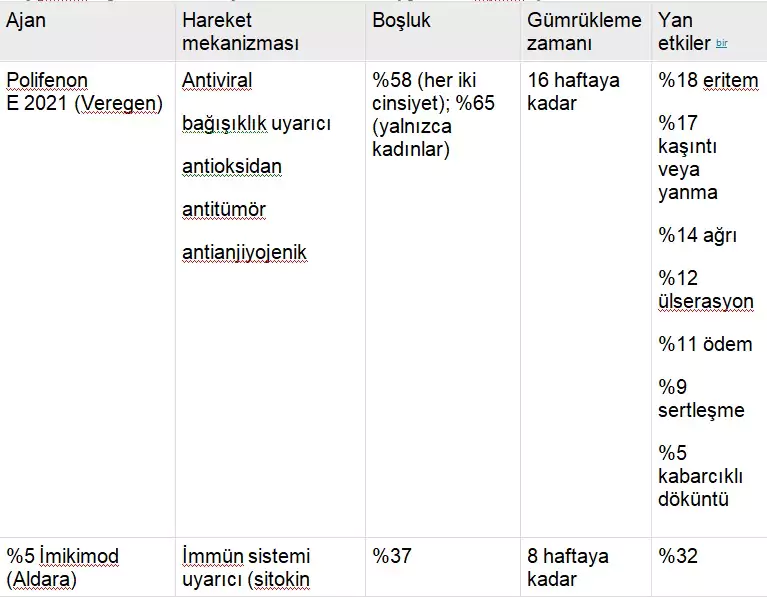
The spectrum of biological activities of green tea catechins is shown in Table 1. In addition to their antiviral properties and immune-stimulating characteristics, catechins also exhibit antitumor and antioxidant effects. There is also sufficient evidence for their antiangiogenic activity.
TABLE 1
Biological Properties of Green Tea Catechins

Antiviral Properties
In its episomal form, the early gene E2 of HPV prevents the transcription of viral oncogenes E6 and E7, leading to a non-malignant phenotype. However, integration into the host genome results in the disruption of E2 and thus the loss of suppression of E6 and E7, causing the disruption of p53 and pRB (retinoblastoma) signaling and oncogenesis. The potential for integration and thus malignant transformation seems to vary among different HPV types; HPV types 16 and 18 are most commonly found in invasive lower genital tract cancers, while HPV types 6 and 11 are associated with over 90% of genital warts. As discussed later, catechins have demonstrated clinical efficacy in the treatment of anogenital warts. This activity may be distinct from their antiviral properties.
Hastak et al. demonstrated that apoptosis induced by EGCG in human prostate carcinoma cells is mediated through the stabilization of p53 via phosphorylation on critical serine residues. Khafif et al. examined the effects of EGCG on an oral leukoplakia cell line and noted growth inhibition induced through a G1 block with an increase in the under-phosphorylated form of pRB. Given the stabilization of two key tumor suppressor gene products, which are targets of HPV-mediated transformation, catechins are thus hypothesized to enhance cell cycle regulation and inhibit HPV-induced cellular growth. Currently, there is no evidence that catechins directly interact with HPV oncogenic products E6 and/or E7.
Anti-inflammatory and Immune-Stimulating Properties
The anti-inflammatory activity of green tea catechins can largely be attributed to their antioxidant effects. EGCG has been found to inhibit the activity of transcription factors AP-1 and NF-kappa B, both of which can mediate many inflammatory processes and can be activated by reactive oxygen species.
While Langerhans cells are typically the first antigen-presenting cells to encounter infectious viruses in humans, these immune mediators are not activated by HPVs. However, catechins induce the release of many immune-stimulating interleukins while suppressing the release of immune-inhibiting interleukins. Specifically, catechins activate T lymphocytes, induce the release of both tumor necrosis factor-alpha (TNF-a) and interferon-gamma (IFN-y), and stimulate macrophages to release immune-stimulating cytokines. This promotes the recruitment of monocytes, dendritic cells, lymphocytes, natural killer cells, and T-helper cells to aid the immune response (Figure 3).
Epithelial Hyperplasia and Neutrophil Infiltration in Mouse Tissue
Epithelial hyperplasia and neutrophil infiltration were noted in mouse tissue following the topical application of 15% Polyphenon E ointment.
Meltzer. Green Tea Catechins for the Treatment of External Genital Warts. Am J Obstet Gynecol 2009.
Since interleukin-8 (IL-8), a major neutrophil chemoattractant and inflammatory mediator, is dependent on IL-1β through the inhibition of IL-1β, catechins reduce the recruitment of neutrophils. Catechins also downregulate CF8+ T cells and inhibit cyclooxygenase-2 (COX-2). The downregulation of the immune system through COX-2-mediated prostaglandin E2 (PGE-2) expression in epithelial cells has been associated with the development of dysplasia. Finally, at low doses, catechins stimulate mitogen-activated protein kinase (MAPK) pathways, thereby increasing the transcription of proto-oncogenes c-jun and c-fos. At higher doses, catechins lead to apoptosis by activating c-jun N-terminal kinase (JNK), a member of the MAPK family.
Clinical Trials of Catechins for the Treatment of Anogenital Warts
It is estimated that 75-80% of sexually active adults will contract a genital HPV infection by the age of 50. In 2000, the first age- and gender-specific cost estimates for anogenital warts were made. Among privately insured patients with an insurance claim, each case of genital warts resulted in an average of more than 3 doctor visits and $436 in costs.
Condylomas are typically asymptomatic but can present with itching, pain, bleeding, burning, or vaginal discharge. They appear skin-colored and can be smooth papules or papilliform. Treatment consists of chemical destruction, immunological therapy, or surgical excision; however, 20-30% regress spontaneously. Chemical therapy aims to induce cell death. Podofilox halts the tumor cell cycle, trichloroacetic acid (TCA) causes protein coagulation, and fluorouracil interferes with DNA synthesis. Immunological therapy, such as imiquimod, induces cytokines. Surgery, via cryotherapy, laser ablation, or excisional treatment, is reserved for condylomas resistant to other treatment methods. While each of these treatment methods can be effective in some patients, none are free from side effects and sequelae.
Three randomized, double-blind, placebo-controlled phase 3 trials were conducted to determine the efficacy and safety of catechin Polyphenon E, provided as 10% and 15% ointments. These studies were powered to demonstrate the superiority of each of the 2 Polyphenon E ointment formulations over placebo in terms of complete clearance rates of all baseline and new warts.
The First Study (CT 1015)
Gross et al. conducted the first study in 2007, enrolling patients from 28 hospitals and clinical offices in Germany and Russia. A total of 125 men and 117 women were randomly assigned to Polyphenon E 15% ointment, 10% ointment, or placebo. Treatment was administered for up to 12 weeks with a subsequent 12-week treatment-free follow-up. For the 15% ointment, statistically significant differences in the complete clearance of all baseline external genital warts compared to placebo were achieved (61.0% vs. 40.5% in men, 56.8% vs. 34.1% in women; combined: P = .0066). For the 10% ointment, 53.8% of men and 39.5% of women achieved complete clearance. Recurrence rates 12 weeks after the end of treatment ranged from 10.3% to 11.8% across the three groups. Nineteen patients (7.9%) experienced a total of 21 adverse events: seven in the 10% ointment group, nine in the 15% ointment group, and three in the placebo group. Only six patients (2.5%) had toxicities considered probably or possibly related to the study drug (hyperkeratosis and skin discoloration in two patients with 10% ointment, and temporary local necrosis, allergic dermatitis, or foreskin pain in four patients with 15% ointment).
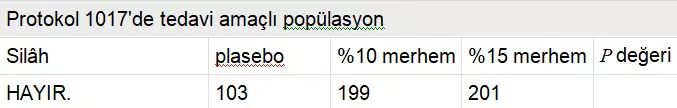
The Second Study (CT 1017)
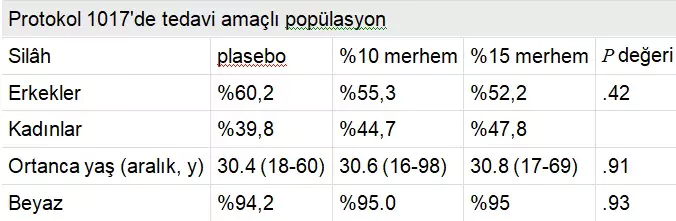
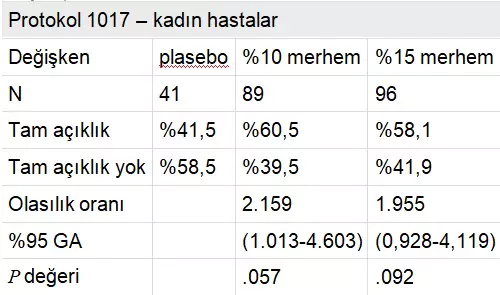
Protocol AG CT 1017 was a randomized, double-blind, three-arm parallel group, vehicle-controlled, multicenter phase 3 study enrolling 226 women and 277 men from 46 dermatological, gynecological, and urological centers in Europe and South Africa. The placebo-controlled trial investigated the 15% and 10% Polyphenon E ointments, with results recently reported by Stockfleth et al. The median age of the study population was approximately 30.7 years (range, 16-98 years), with over 90% of subjects in each of the three arms being white (Table 2). The median wart area was 51 mm² and the median number of warts was six. Over 50% clearance was achieved in 77.3% of subjects in the 15% ointment group, 78.0% in the 10% ointment group, and 52.9% in the placebo group. Approximately 60% of women and 45% of men in both active groups achieved complete clearance of all warts, indicating that women responded better than men. The estimated median time to complete clearance was 16.1 weeks for both the 15% and 10% ointments and 16.7 weeks for placebo (P < .001). Adverse events, excluding mild local reactions possibly related to the study drug, were reported by four patients (0.8%) in the Polyphenon E 15% ointment group, including moderate balanitis, severe herpes simplex, mild lymphadenitis, and severe phimosis.
TABLE 2
TABLO 3
Üçüncü deneme, AG Protokolü CT 1018, 502 deneği (258 erkek ve 244 kadın) %15 merhem, %10 merhem ve araç kollarına rastgele atadı ve Amerika Birleşik Devletleri, Latin Amerika ve Romanya’daki 50 sağlık merkezinde gerçekleştirildi. . 21 %15 merhem kolunda yüz on bir kişi (%57,2), %10 merhem kolunda 111 kişi (%56,3) ve araç kolunda 35 kişi (%33,7) tüm dış genital siğillerin tamamen temizlenmesini sağladı ( P < .001). 21 Erkek hastalarla karşılaştırıldığında, tüm siğillerden tamamen temizlenen kadın hastaların oranı 3 tedavi grubunun hepsinde daha yüksekti. 12 haftalık takip süresi boyunca 3 kolun tamamında hastaların %6,5 ila %8,8’inde nüks meydana geldi. %15 merhem ile tedavi edilen 5 hastada ve %10 merhem ile tedavi edilen 2 hastada lenfadenit, deri ülseri, vulvit ve vulvovajiniti içeren ciddi advers olaylar bildirilmiştir. 21
3 çalışmanın her birinde, plasebo kollarında siğillerden tamamen kurtulan hastalar vardı. Yüksek plasebo yanıtının 2 yardımcı maddenin bilinen potansiyeline bağlı olma olasılığı vardır: oleil alkol ve izopropilmiristat (IPM). Daha da önemlisi, IPM’nin bilinen bazı tahriş edici potansiyeli vardır ve mekanik uyaran ve hijyen değişikliği ile birlikte günde 3 kez uygulamanın daha yüksek spontan klerense yol açabileceği düşünülmektedir. Çalışmaların 2’sinde (AG 1017 ve 1018), cinsiyet farklılıkları nispeten küçük olmasına rağmen, sinecatechins pomad için tam klerens oranlarının kadınlarda erkeklerden daha yüksek olması da ilginçtir. Erkeklerde daha düşük klirens oranları, penil şaft boyunca derinin daha fazla keratinleşmesiyle bağlantılıdır ve bu da ilaç penetrasyonunu etkiler. 22 Polyphenon E %15 merhem ile topikal tedaviden sonra vulvar kondilomun progresif rezolüsyonunu ve temizlenmesini gösteren bir vaka çalışması Şekil 4’te görülmektedir .
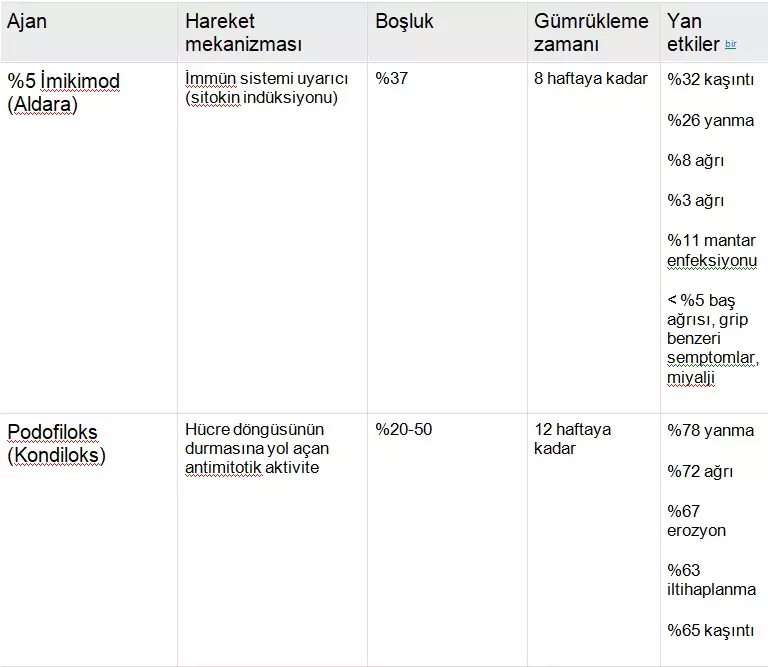
Tablo 4 yeşil çay kateşinleri ve anogenital siğillerin tedavisi için FDA tarafından onaylanan diğer 2 hasta tarafından uygulanan topikal ajanla ilişkili özellikleri, aktiviteyi ve yan etkileri listeler: podofiloks ve imikimod. Podofillotoksin (podofilox), lignan ailesinden alkaloid olmayan bir toksindir ve genital siğillerin tedavisi için FDA onaylı ilk jeldir. Tyring ve diğerleri 23 çift kör, araç kontrollü, çok merkezli bir çalışmada anogenital siğilleri olan 326 hastanın sonuçlarını bildirmiştir. Tedavi amaçlı analizde, 8 haftalık tedaviden sonra, %0,5 podofilox jel ile tedavi edilen 167 hastanın 62’sinde (%37,1) tedavi edilen alanlardan tamamen temizlenme olurken, siğillerden kurtulan 86 hastanın 2’sinde (%2,3) araç jeli ile ( P < .001). 23 %0,5 podofiloks jeli iyi tolere edildi ve ilaca bağlı lokal reaksiyonlar nedeniyle sadece 7 hasta (%3,2) çalışma tedavisini bıraktı. 23 Pazarlanan ticari isim ürünü Condylox, %0.5’lik bir podofilox jelidir.
TABLO 4
As in an FDA Package Insert
Imiquimod has the ability to induce various cytokines, including INF-a, TNF-a, and also IL-1, -6, and -8. Beurner et al. conducted a prospective, double-blind, placebo-controlled study with random assignment of 51 subjects to the imiquimod 5% arm and 57 to placebo, totaling 108 subjects. It was reported that 37% of patients treated with imiquimod and 0% of the placebo group achieved complete clearance of warts (P < .001). Among the 16 patients who achieved complete clearance, three (19%) experienced recurrence during the 10-week follow-up period. Subjects in the imiquimod arm experienced significantly more local inflammatory reactions ranging from pain and ulceration (8.3-10.4%) to itching (54.2%). Imiquimod 5% received FDA approval in 1997 and is marketed under the trade name Aldara by MEDA AB, Graceway Pharmaceuticals, and iNova Pharmaceuticals. Although the clearance rates listed in Table 4 suggest higher rates for Polyphenon E preparations compared to podofilox and imiquimod, based on current clinical trials, the clearance duration for Polyphenon E is estimated to be up to 16 weeks, compared to 8 weeks (imiquimod) and 12 weeks (podofilox).
Summary
The FDA approval of Gardasil, a quadrivalent HPV 6/11/16/18 recombinant virus-like particle vaccine (Merck & Company, Inc, Whitehouse, NJ), may lead to significant advances in the eradication of HPV-associated diseases, including invasive cervical cancer, lower genital tract dysplasias, and condyloma. However, it is estimated that these benefits will not occur for at least one full generation, assuming, of course, that mass vaccination campaigns are successful globally. Until then, anogenital warts will continue to cause significant morbidity for millions of Americans. Until recently, most existing treatments have been only moderately effective. Randomized trials have demonstrated the efficacy of green tea catechins as a topical treatment for anogenital warts with negligible toxicity. Polyphenon E (Veregen) ointment not only becomes the first botanical approved for the treatment of anogenital warts but also the first botanical approved by the FDA under current regulatory policy. Polyphenon E (Veregen) ointment received FDA approval on October 31, 2006, and is protected by patent until 2017. The wholesale price of Veregen for pharmaceutical distributors is $208.98 per 15 g tube. The addition of green tea catechins to the armamentarium provides a new topical agent that can be used as a first-line treatment for patients or before surgical treatment in case of recurrence of anogenital warts after other topical therapies.
The article should be cited as follows: Meltzer SM, Monk BJ, Tewari KS. Treatment of external genital warts with green tea catechins. Am J Obstet Gynecol 2009;200:233.e1-233.e7. Reprints cannot be obtained from the authors.
References
- 1. Monk BJ, Tewari KS: İnsan papilloma virüsü enfeksiyonunun spektrumu ve klinik sekelleri. Gynecol Oncol 2007; 107: s. S6-S15.
- 2. Demeule M., Michaud-Levesque J., Annabi B., et. al.: Yeni antitümör ve antianjiyojenik bileşikler olarak yeşil çay kateşinleri. Curr Med Chem Antikanser Ajanları 2002; 2: s. 441-463.
- 3. Cooper R., Morre DJ, Moore DM: Yeşil çayın tıbbi faydaları: bölüm 1, kanser dışı sağlık yararlarının gözden geçirilmesi. J Alternative Complim Med 2005; 11: s. 521-528.
- 4. Nagle DG, Ferreira D., Zhou YD: Epigallocatechin-3-gallate (EGCG): kimyasal ve biyomedikal perspektifler. Fitokimya 2006; 67: s. 1849-1855.
- 5. Cabrera C., Artacho R., Gimenez R.: Yeşil çayın faydalı etkileri–bir inceleme. J Am Col Beslenmesi 2006; 25: s. 101-1 79-9
- 6. Tewari KS, Taylor JA, Liao SY, et. al.: Şiddetli bir kombine immün yetmezlik murin-insan ksenogreft modelini kullanan genel bir servikal karsinojenez teorisinin geliştirilmesi ve değerlendirilmesi. Jinekol Oncol 2000; 77: s. 137-148.
- [ PMC ücretsiz makale ] [ PubMed ] 7. Hastek K., Gupta S., Ahmad N., Agarwal MK, Agarwal ML, Mukhtar H.: LNCaP’de epigallocatechin-3-gallate-indüklenmiş apoptozda p53 ve NF-KB’nin rolü hücreler. Onkojen 2003; 22: s. 101-1 4851-4
- 8. Khafif A, Schantz SP, Al-Rawi M, Edelstein D, Sacks PG: Yeşil çay, oral lökoplakide hücre döngüsü ilerlemesini düzenler. Baş Boyun 1998; 20: s. 101-1 528-534.
- 9. Ahn WS, Huh SW, Bae S.-M., et. al.: Yeşil çayın önemli bir bileşeni olan EGCG, apoptoz, G1 tutuklaması ve gen ekspresyonunun düzenlenmesi yoluyla bir insan rahim ağzı kanseri hücre dizisi olan CaSki hücrelerinin büyümesini engeller . DNA Hücre Biol 2003; 22: s.217-224.
- 10. Ahn W.-S., Yoo J., Huh S.-W., et. al.: Yeşil çay özlerinin (polifenon E ve EGCG) insan servikal lezyonları üzerindeki koruyucu etkileri. Eur J Cancer Önceki 2003; 12: s. 383-390.
- 11. Sutherland BA, Rahman RM, Appleton I.: İskemi kaynaklı nörodejenerasyona odaklanan yeşil çay kateşinlerinin etki mekanizmaları. J Nutr Biochem 2006; 17: s. 291-306.
- 12. Rahman I., Biswas SK, Kirkham PA: Diyet polifenolleri tarafından iltihaplanma ve redoks sinyalinin düzenlenmesi. Biochem Pharmacol 2006; 72: s. 1439-1452.
- 13. Zaveri NT: Yeşil çay ve polifenolik kateşinleri: kanser ve kanser dışı uygulamalarda tıbbi kullanımlar. Hayat Bilimi 2006; 78: s. 2073-2080.
- [ PMC ücretsiz makale ] [ PubMed ] 14. Won S.-M., Park Y.-H., Kim H.-J., Park K.-M., Lee W.-J.: Kateşinler anjiyotensin II’yi inhibe eder- mitojenle aktive olan protein kinaz yolu aracılığıyla vasküler düz kas hücresi proliferasyonunu indükledi. Uzman Molec Med 2006; 38: s. 101-1 525-534.
- 15. Shimizu M., Weinstein IB: Çay kateşinleri ve ilgili fitokimyasallar tarafından sinyal transdüksiyonunun modülasyonu. Mutasyon Res 2005; 591: s. 147-160.
- 16. Kao YH, Hiipakka RA, Liao S.: Endokrin sistemlerin modülasyonu ve yeşil çay epigallocatechin gallate ile gıda alımı. Endokrinoloji 2000; 141: s. 141-1 980-9
- 17. Higdon JV, Frei B.: Çay kateşinleri ve polifenoller: sağlık etkileri, metabolizma ve antioksidan fonksiyonlar. Crit Rev Food Sci Nutr 2003; 43: s. 89-143.
- 18. Insinga RP, Dasbach EJ, Myers ER: Amerika Birleşik Devletleri’ndeki bir dizi özel sağlık planında genital siğillerin sağlık ve ekonomik yükü. Clin Infect Dis 2003; 36: s. 1397-1403.
- 19. Gross G., Meyer K.-G., Pres H., Thielert C., Tawfik H., Mescheder A.: A randomize, çift kör, dört kollu paralel grup, plasebo kontrollü faz II/III Polifenon E’nin iki galenik formülasyonunun dış genital siğillerin tedavisinde klinik etkinliğini araştırmak için yapılan çalışma. J Eur Acad Dermatol Venereol 2007; 21: s. 1404-1412.
- 20. Stockfleth E., Beti H., Orasan R., et. al.: Dış genital ve perianal siğillerin tedavisinde Topikal Polifenon E: randomize kontrollü bir çalışma. Br J Dermatol 2008; 158: s. 1329-1338.
- 21. Tatti S., Swinehart JM, Thielert C., Tawfik H., Mescheder A., Beutner KR: Sinecatechins, tanımlanmış bir yeşil çay ekstresi, dış anogenital siğillerin tedavisinde: randomize, kontrollü bir çalışma. Obstet Jinekol 2008; 111: s. 1371-1379.
- 22. Gollnick H., Barasso R., Jappe U., et. al.: Sünnetsiz erkeklerde haftada üç kez veya günde bir kez uygulandığında penis genital siğillerinin tedavisinde imikimod %5 kreminin güvenliği ve etkinliği. Uluslararası J STD AIDS 2001; 12: s. 22-28.
- 23. Tyring S., Edwards L., Cherry LK, et. al.: Anogenital siğillerin tedavisinde %0.5 podofilox jelin güvenlik ve etkinliği. Arch Dermatol 1998; 134: s.33-38.
- 24. Beurner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, Douglas JM: Genital siğillerin bir bağışıklık yanıtı değiştiricisi (imikimod) ile tedavisi. J Am Acad Dermatol 1998; 38: s.230-239.
